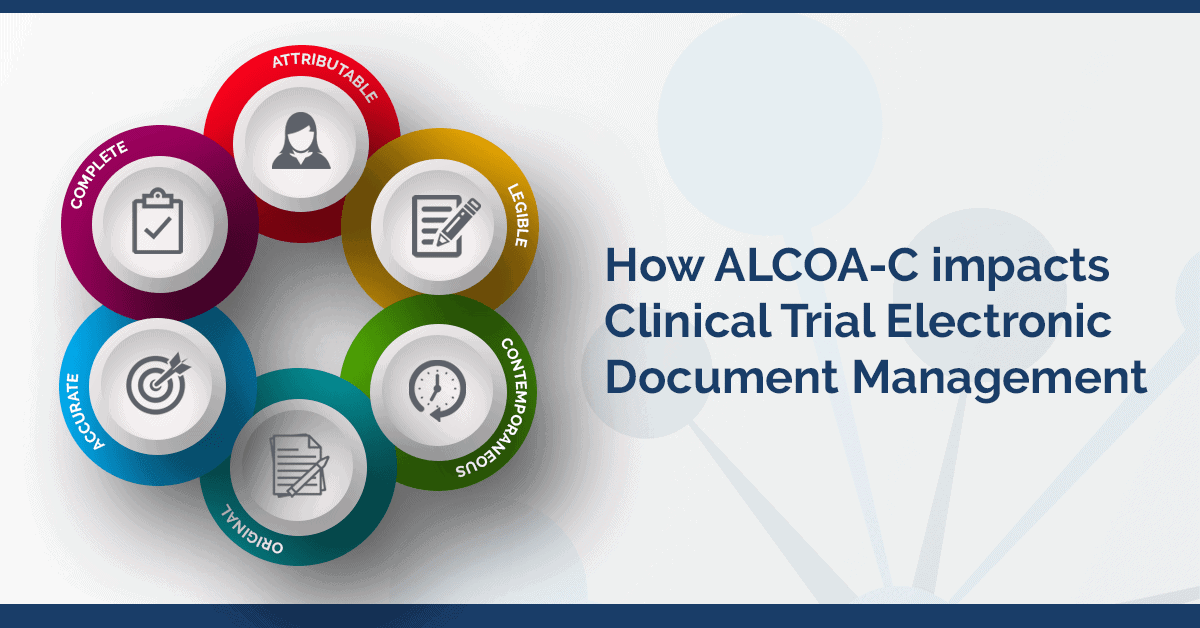
PDF) Research Electronic Data Capture (REDCap) electronic Informed Consent Form (eICF) is compliant and feasible in a clinical research setting

Electronic Source Data in Clinical Investigations: Navigating the Final FDA Guidance Trailer - YouTube
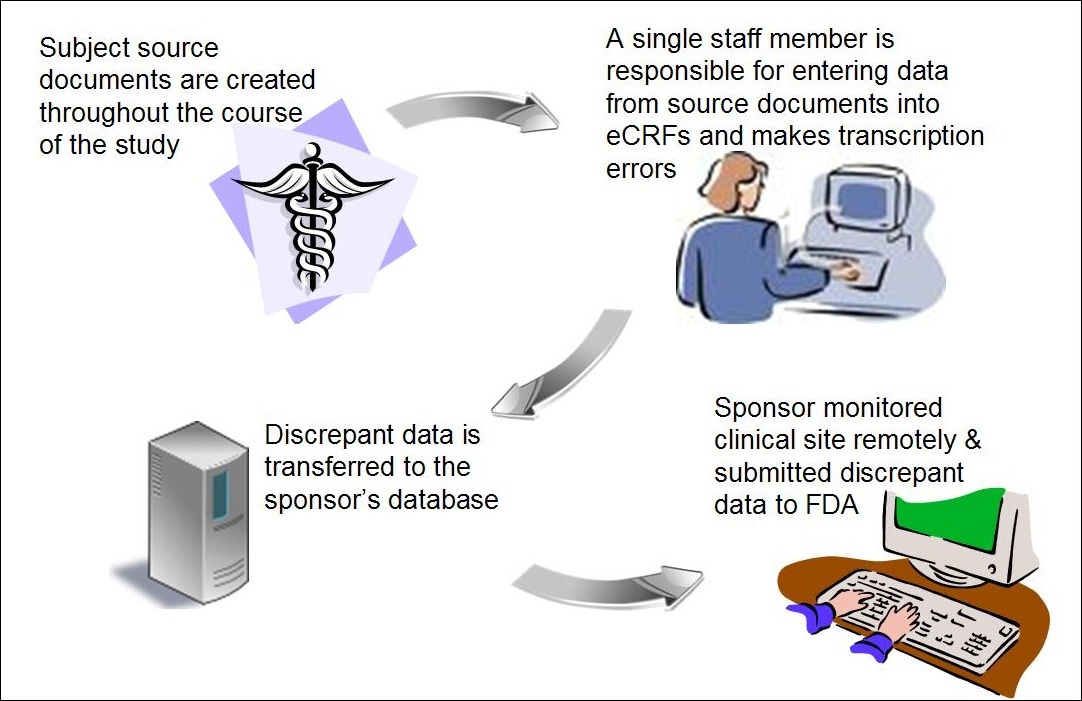
![PDF] Electronic Data Capture in clinical trials– interface design and evaluation and system validation | Semantic Scholar PDF] Electronic Data Capture in clinical trials– interface design and evaluation and system validation | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/50a3193b5f8fb7f91479fd8839c1a6d03baba9b1/19-Figure2-1.png)
PDF] Electronic Data Capture in clinical trials– interface design and evaluation and system validation | Semantic Scholar

Data Management for Pharmaceutical Trials Michael A. Kohn, MD, MPP (Acknowledgment: Susanne Prokscha) - ppt download
Clinical Research Associate (CRA) A Complete Guide on How to Become a Clinical Research Associate — Clinical Research Certification