
US FDA Guidance – Good Clinical Practice: Integrated Addendum to ICH E6(R1) - Lachman Consultant Services, Inc.

ich e6 r1 good clinical practice consolidated guidance — Clinical Research Certification I Blog - CCRPS
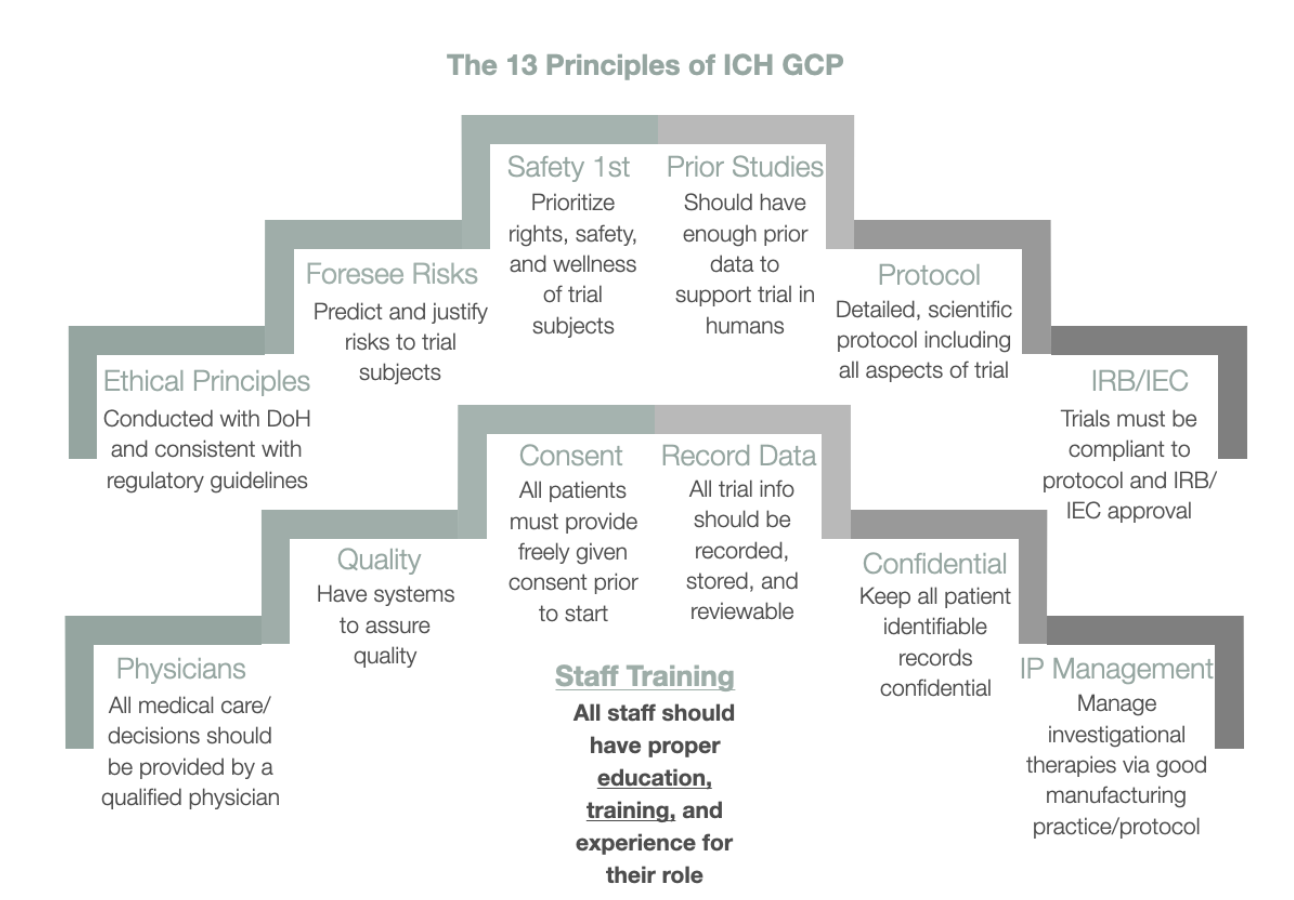
in the united states following the ich e6 guideline is — Clinical Research Certification I Blog - CCRPS

Principles of Good Clinical Practice (GCP) – What is it all about and who is responsible for adherence? GCP and QA All SIAC Call Mar 14, 2008 Munish Mehra, - ppt download
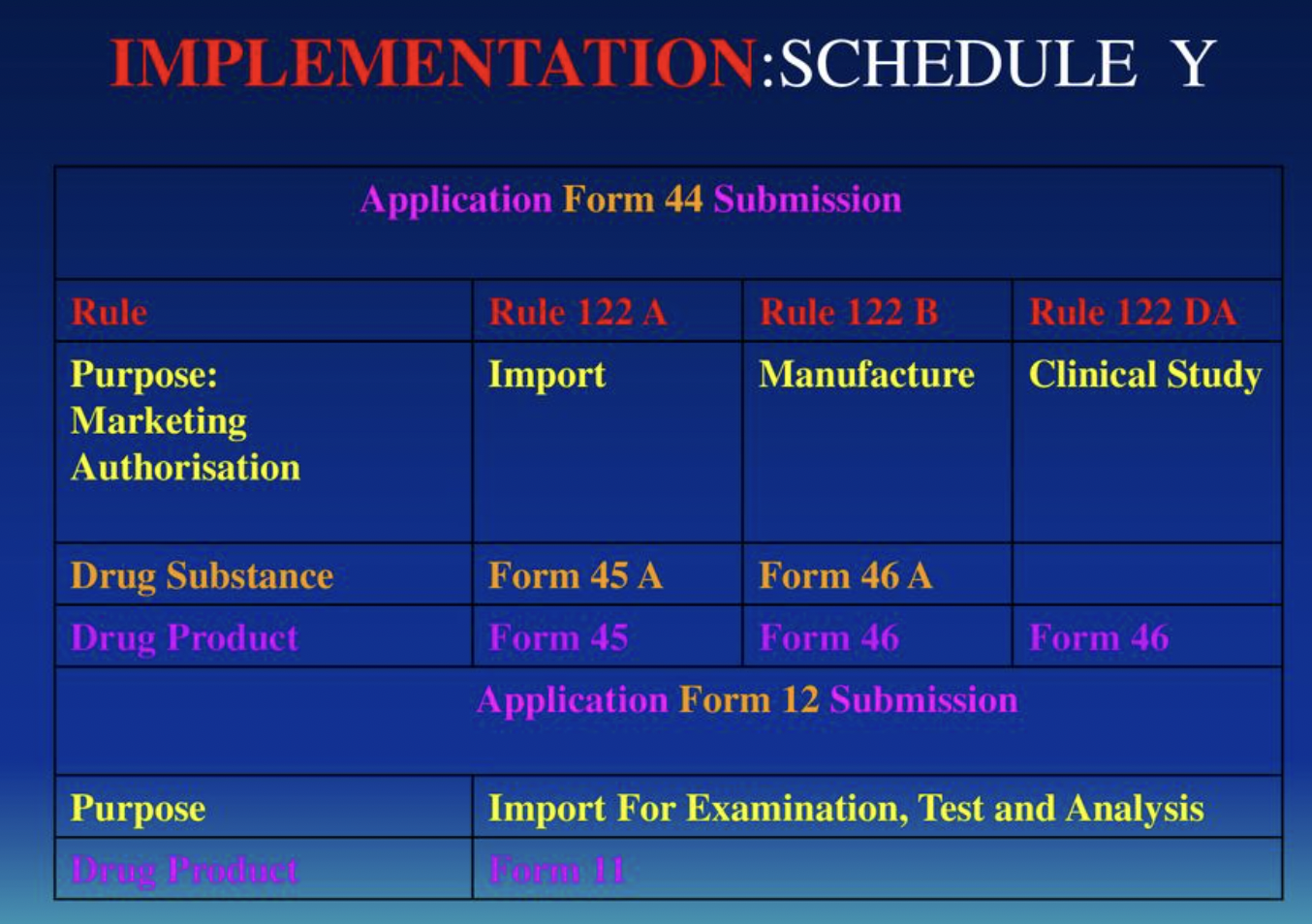
difference between ich gcp indian gcp and schedule y ppt — Clinical Research Certification I Blog - CCRPS

POL-0030: Compliance and enforcement approach and inspection strategy for clinical trials of drugs involving human subjects - Canada.ca

Book M1: 2022 Mini Pocket-Sized (3" x 5") ICH Guidelines for GCP (E6) – Clinical Research Resources, LLC


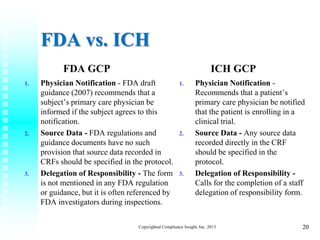
.jpg)










